|
INTRODUCTION
Activated carbon can adsorb an extremely wide range of hazardous compound.
In the present study, we used plasma in order to improve
the adsorption ability of activated carbon. Plasma treatment
probably will gives change to the surface chemical structure and
pore structure, as for these structures
give a big
influence in adsorption property. There are many types of plasma,
but in this research the plasma dielectric barrier discharge was
utilized in generation process. In this treatment process, the
interchange discharge was put between the electrodes. Plasma
usually generates under high temperature and low pressure
conditions, but as for dielectric barrier discharge with the
dielectric current is restricted,
the discharge is maintained to occur at normal temperature
and atmospheric pressure, so it is not necessary to provide the
vacuum devices. Furthermore, the plasma treatment becomes more
possible at low cost
due to small energy. In
this research, the pH and specific
surface area etc are measured in order to know the changes in
surface character by plasma treatment. Furthermore, we used plasma
treated activated carbon on adsorption of
the arsenic and compared
it with the untreated activated carbon.
@
EXPERIMENTAL
METHOD
The apparatus of this experiment is shown in Fig.1.
In the discharge section, two
copper plates were
arranged parallel. As for treatment method, activated carbon were
put between electrodes. Let O2 through and then impressed
the voltage in the reactor. After specified time, stopped
the discharge and then fetched the activated carbon.
Plasma
treatment condition is shown in Table 1. On the other hand, pH 10.38
of arsenic solution is used in adsorption experiment, the adsorption
experimental condition is shown in Table 2.
@
RESULTS
AND DISCUSSION
Chemical property like pH also gives an influence in
adsorption
phenomenon instead of
pore
structure of activated carbon (surface area, pore volume, pore
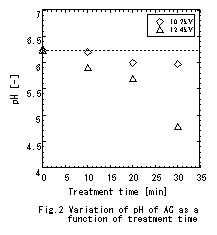
diameter distribution). Fig.2 shows the variation
of pH of activated carbon as a function of treatment time. It can be
concluded that the increase in pH with time is due to the
oxidation
reaction
occurred, as a result of
forming phenol type hydroxyl (- OH) and carboxyl group (-COOH) etc
(from
previous result of low temperature
oxygen plasma
experiment). On the other hand, the specific surface area
did
not change abruptly
even if it
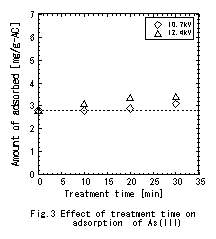
was treated by plasma. The result of adsorption
experiment of arsenic is shown in Fig.3. As Fig.3 shows, the
increase of adsorption quantity is not excessively seeing at 10.7kV,
whereas at 12.4kV, the adsorption quantity is gradually increased
with treatment time. It can be concluded that all the pore volume
was increased.
@
CONCLUSION
As a
conclusion, when the activated carbon is
treated by plasma, the pH of the activated carbon decreased and the mesopore volume increased. This
time, from the result of arsenic adsorption experiment we could see
slightly improvement of adsorption
properties.
@
@
@
@
@
@
@
@
@
@
@
@
@ |
|
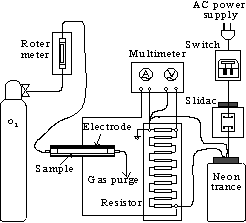
Fig.1
Experimental apparatus
@
@
|
| Discharge voltage |
10.7 - 12.4 kV |
| Plasma gas |
Oxygen |
| Gas flow rate |
0.5 L/h |
| Treatment time |
10 - 30 min |
| Table 1 Plasma treatment
condition |
|
@
| Temperature |
25 |
| Pressure |
Atmospheric
pressure |
| Adsorption time |
6 - 96h |
| Adsorbent weight |
0.1 g |
| Table 2
Adsorption experimental condition ( pH 10.38)
@
|
|
 |
@
|
 |
|
![]()